Enhancing bioavailability of your molecule
On top of the lack of stability of biological agents in the gastrointestinal tract, their capacity to reach the bloodstream from the gut is also an important challenge, making them unsuitable for per-os administration for a systemic effect.
Affilogic is developing a platform to enhance bioavailibility of a molecule fused to a Nanofitin®-based shuttle after oral administration. The mechanism of action relies on a receptor-mediated transcytosis, like for the BBB-crossing platform currently developed by Affilogic and its partners.
 The objectives of this Gut-To-Blood (G2B) platform are:
The objectives of this Gut-To-Blood (G2B) platform are:
-To enhance bioavailibility of a partner’s molecule, provided that such molecule is formulated to be compatible with an oral route
 -To engage our discovery process and design custom Nanofitins® for our partner, with a therapeutic activity in the bloodstream. The lead candidate will be fused to the G2B shuttle for an active transport in the vascular compartment after oral route.
-To engage our discovery process and design custom Nanofitins® for our partner, with a therapeutic activity in the bloodstream. The lead candidate will be fused to the G2B shuttle for an active transport in the vascular compartment after oral route.
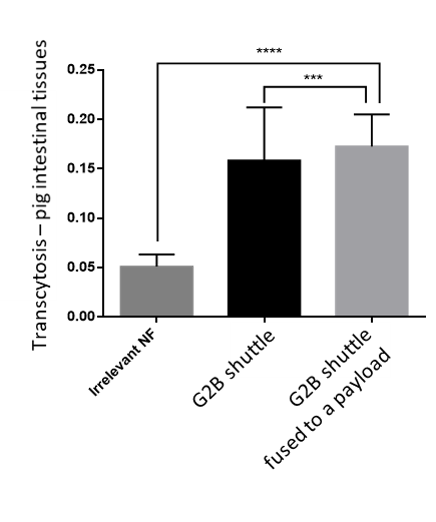
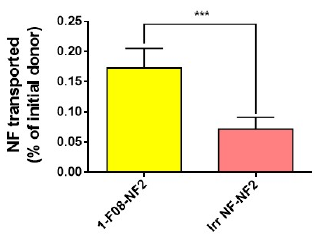 A first publication showed that an anti-LepR Nanofitin® allows efficient Gut-Crossing, through Receptor Mediated Transcytosis, of another active Nanofitin® in an ex-vivo procine intestinal model (Masloh et al., Enhancing Oral Delivery of Biologics: A Non-Competitive and Cross-Reactive Anti-Leptin Receptor Nanofitin Demonstrates a Gut-Crossing Capacity in an Ex Vivo Porcine Intestinal Model, Pharmaceutics 2024), opening the door to systemic delivery of biologics after oral administration.
A first publication showed that an anti-LepR Nanofitin® allows efficient Gut-Crossing, through Receptor Mediated Transcytosis, of another active Nanofitin® in an ex-vivo procine intestinal model (Masloh et al., Enhancing Oral Delivery of Biologics: A Non-Competitive and Cross-Reactive Anti-Leptin Receptor Nanofitin Demonstrates a Gut-Crossing Capacity in an Ex Vivo Porcine Intestinal Model, Pharmaceutics 2024), opening the door to systemic delivery of biologics after oral administration.
The G2B project, conducted by Affilogic and its partners Artois University (France), Geneva University (Switzerland) and Eindhoven University (Netherlands), is partially sponsored from November 2020 to October 2023 by a grant from Eurostars / the European Commission via BPI France (DOS0130843/00).

