Proteins from the sac7d family
Sac7d is a protein of 66 amino acids (7 kDa) constituted of a single chain folded as an OB-fold (oligonucleotide/oligosaccharide-binding-fold), a β-barrel capped by a C-terminal α-helix, and lacking disulphide bridges. Sac7d belongs to a class of small chromosomal proteins from the hyper thermophilic archaeon Sulfolobus acidocaldarius. This protein, discovered in 1974 in Yellowstone National Park geysers, is extremely stable to heat and acid (natural environment 85°C and pH 2). Often described as a histone-like protein, Sac7d binds to DNA without any particular sequence preference resulting in an increase of the DNA melting temperature by approximately 40 °C, thereby protecting the genome from thermal denaturation.
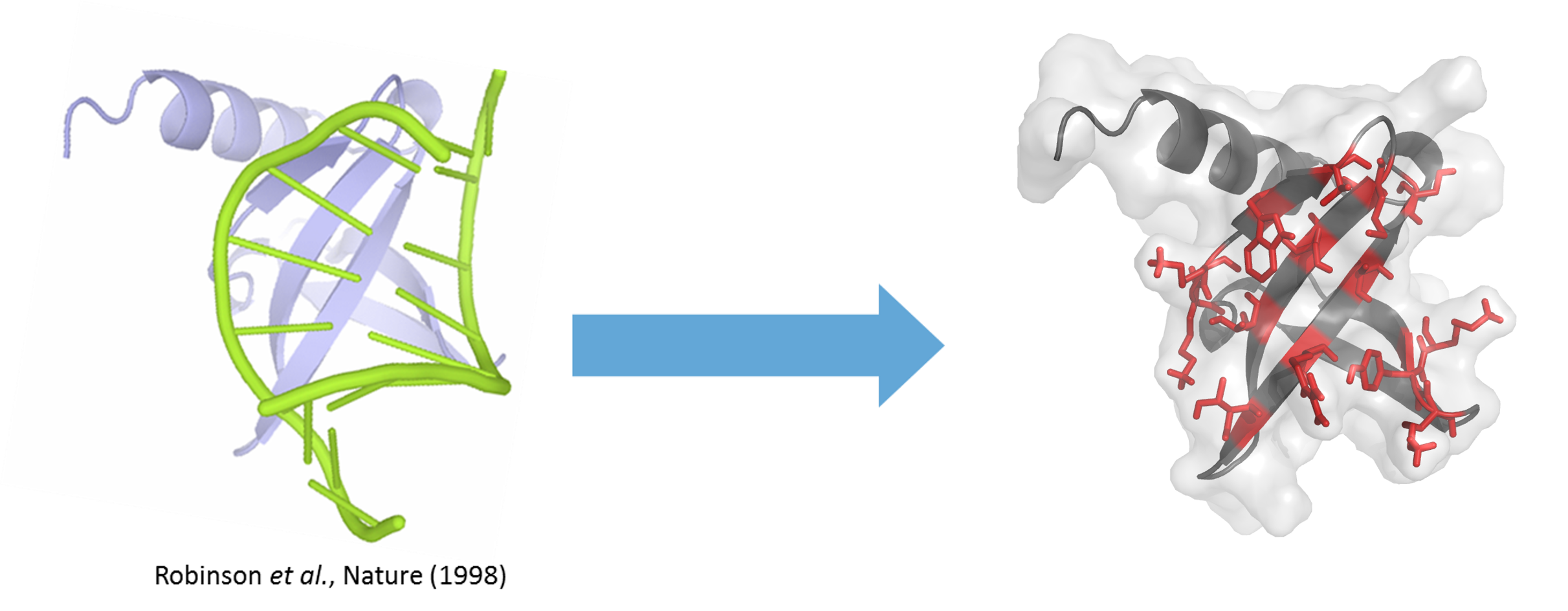
From natural proteins to efficient therapeutics
Reengineering of the natural binding site by randomisation of the residue exposed to the solvent (residue in red on figure) allows the creation of libraries of Nanofitin® variants from the wild-type Sac7d protein. Libraries, involving up to 20% of the total residue, are designed so as to fully redirect the molecular recognition against any target presented, with the result that Nanofitins® no longer recognise DNA. Such Nanofitins® combine both a specific, high affinity binding on the target and conservation of the original stability features, i.e. being thermophilic and acidophilic.
This technology resulted in the creation of a proprietary discovery process, exclusively performed by Affilogic and open for external collaborations.
